WATER HARDNESS
-
The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Hard water is high in dissolved minerals, largely calcium and magnesium.
-
Hard water can cause mineral buildup in plumbing, fixtures, and water heaters, and poor performance of soaps and detergents. When using hard water, more soap or detergent is needed to get things clean, be it your hands, hair, or laundry. And the scale will reduce the life of equipment, raise the costs of heating the water, lower the efficiency of electric water heaters, and clog pipes. The mineral buildup will occur in your home coffee maker, too…
-
Considering hard water can pose critical problems in equipment that handles water, it is necessary to monitor to avoid risk of costly breakdowns.
-
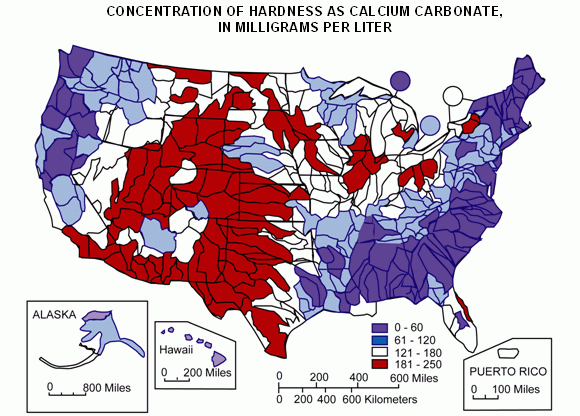
Hardness is caused by compounds of calcium and magnesium, and by a variety of other metals. General guidelines for classification of waters are: 0 to 60 mg/L (milligrams per liter) as calcium carbonate is classified as soft; 61 to 120 mg/L as moderately hard; 121 to 180 mg/L as hard; and more than 180 mg/L as very hard.
-
Below is map of water hardness in the United States FYI.
-
You must need water treatment & filter accessories to reduce water hardness.
•References: U.S. Geological Survey

